Table of Contents
Epidiolex
Overview of Epidiolex: An Introduction to the FDA-Approved Drug

A notable advance in medical treatment, especially for certain rare and severe epileptic disorders, comes with the introduction of the first cannabinoid-based drug approved by the U.S. Food and Drug Administration (FDA). This comprehensive overview delves into the development, pharmacological attributes, clinical applications, and the regulatory impact of this pioneering medication.
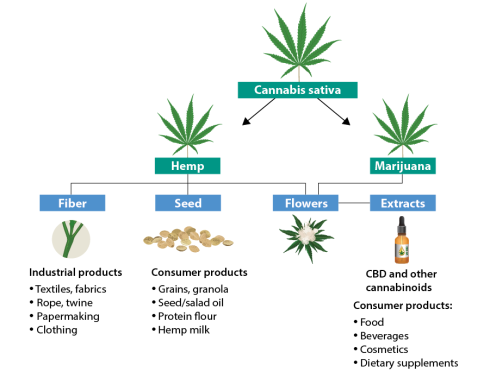
Development and Composition: The drug, consisting of cannabidiol (CBD), a non-psychoactive compound extracted from the Cannabis sativa plant, was developed by GW Pharmaceuticals. It underwent extensive clinical testing to validate its safety and effectiveness, marked by its lack of psychoactive effects, distinguishing it from other cannabis-related products.
Pharmacological Properties: As a highly purified, plant-derived CBD extract, the drug interacts with the endocannabinoid system, though its precise mechanism of action is not fully understood. It is believed to alter neural signaling systems involved in managing seizure activities.
FDA Approval and Clinical Indications: The drug received FDA approval in June 2018 for the management of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients two years of age and older. This approval was historic, marking it as the first FDA-approved medication containing a purified substance derived from marijuana. Its indications were later extended to include managing seizures associated with tuberous sclerosis complex.
Clinical Efficacy and Administration: Clinical trials demonstrated that the medication significantly reduces seizure frequency in patients with the specified epileptic syndromes, particularly where other treatments have failed. It is administered orally in a liquid form, allowing dosage adjustments based on the patient’s weight and specific health needs.
Regulatory and Legal Impacts: Its approval also impacted drug scheduling standards, leading to the reclassification of FDA-approved drugs containing CBD with less than 0.1% THC as Schedule V drugs, noted for their lower potential for abuse. This reclassification has significant implications for the classification of CBD and illustrates changes in the regulatory framework for cannabis-derived substances.
Impact and Prospects for Future Research: The success of this medication is expected to spur further research into the therapeutic potentials of cannabinoids, laying a foundation for continued cannabinoid research and potentially influencing future pharmaceutical developments that utilize compounds derived from cannabis.
In summary, this FDA-approved cannabinoid medication has significantly broadened the treatment landscape for severe and rare epileptic disorders, marking a critical milestone in the acceptance and integration of cannabinoid-based treatments in healthcare, underscored by extensive clinical evidence of its benefits and safety.
The Active Ingredient in Epidiolex: Cannabidiol (CBD) Explained
Cannabidiol (CBD), the primary active component in Epidiolex, has captured the interest of the pharmaceutical and healthcare industries due to its therapeutic efficacy and absence of psychoactive effects. This comprehensive overview delves into CBD’s role as the essential ingredient in Epidiolex, the first FDA-approved medication derived from cannabinoids, designed to treat certain rare epileptic disorders. This analysis covers CBD’s chemical properties, its pharmacological mechanisms, and its broader medical implications.
- Chemical Properties and Origins: CBD, a non-psychoactive cannabinoid from the Cannabis sativa plant, differs significantly from tetrahydrocannabinol (THC) in its effects. It features a 21-carbon terpenophenolic structure, which plays a critical role in its therapeutic actions and its interaction with the human endocannabinoid system (ECS).
- Pharmacodynamics and Action Mechanism: Unlike other cannabinoids that primarily bind to cannabinoid receptors in the ECS, CBD operates through a variety of non-cannabinoid receptors and ion channels. It also acts to enhance endogenous cannabinoid activity by reducing their breakdown and uptake, showcasing a complex mechanism that underpins its various effects.
- Therapeutic Benefits of CBD: Among its many therapeutic properties, CBD is notably effective as an anticonvulsant, which is the basis for its use in Epidiolex for treating severe epileptic conditions like Dravet syndrome and Lennox-Gastaut syndrome. Research also points to its potential anti-inflammatory, anxiolytic, and neuroprotective effects, which are subjects of ongoing studies.
- Clinical Efficacy of CBD in Epidiolex: In its role within Epidiolex, CBD has demonstrated significant efficacy in reducing seizure frequency in patients, providing an effective treatment option where others have failed. Its inclusion in an FDA-approved drug underscores its medical viability and supports further investigation into cannabinoid-based therapies.
- Regulation and Safety Considerations: The FDA approval of Epidiolex marked a regulatory milestone for cannabis-derived medications, highlighting CBD’s safe profile at administered dosages. However, potential side effects, including gastrointestinal issues and drug interactions, require careful management in clinical use.
- Ongoing Research and Future Prospects: The clinical success of CBD in Epidiolex has spurred additional research into its potential medical applications, leading to a range of clinical trials exploring other therapeutic areas. This continued research aims to deepen understanding of CBD’s pharmacological properties and to expand its clinical applications.
In summary, CBD’s role as the active ingredient in Epidiolex marks a significant advancement in medical therapeutics, especially in the treatment of complex epileptic disorders. The development of Epidiolex as a cannabinoid-based medication highlights the therapeutic possibilities of cannabinoids and underscores the importance of ongoing research and adaptive regulatory approaches to fully realize CBD’s potential in healthcare.
Clinical Trials and Research: The Evidence Behind Epidiolex
Research Overview and Methodology: The approval of this medication was predicated on extensive, multi-stage clinical trials, primarily randomized, double-blind, placebo-controlled studies considered the pinnacle of clinical testing standards. These trials included patients diagnosed with the targeted epileptic syndromes, particularly those who had not benefitted sufficiently from existing treatments.
Dravet Syndrome:
Lennox-Gastaut Syndrome: Several trials targeting Lennox-Gastaut syndrome demonstrated that the medication significantly reduced the frequency of drop seizures, proving its effectiveness over a 14-week period in comparison to a placebo.
Tuberous Sclerosis Complex: In studies involving patients with tuberous sclerosis complex, the medication was more effective in reducing seizure frequency than a placebo, highlighting its potential benefits for this additional condition. In a key trial involving 120 children and young adults with Dravet syndrome, the medication, when used in combination with other antiepileptic drugs, significantly decreased the median frequency of convulsive seizures over a 14-week treatment period compared to a placebo.
Safety Profile and Adverse Effects: The medication was generally well-tolerated. Common side effects included drowsiness, decreased appetite, diarrhea, and elevated liver enzymes, all of which were generally mild to moderate in severity and manageable with medical oversight. The trials also emphasized rigorous monitoring of liver function to address potential liver-related risks.
FDA Approval and Its Significance: The consistently favorable results from these trials led to the FDA’s 2018 approval of the drug. This approval not only provided a new therapeutic option for those with difficult-to-treat epileptic disorders but also established a regulatory framework for the approval of future cannabis-derived pharmaceuticals.
Ongoing Studies and Broader Clinical Applications: Research efforts following FDA approval continue to explore expanding the indications for this medication and investigating its long-term safety profile. The initial success has spurred further exploration into the broader therapeutic possibilities of CBD and related compounds.
In summary,
The clinical trials and ongoing research have solidly positioned this medication as an effective and safe treatment option for Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex, establishing its importance in the realm of medical treatment for epilepsy.
FDA Approval Process: How Epidiolex Became a Recognized Treatment
The approval of Epidiolex by the U.S. Food and Drug Administration (FDA) represents a landmark achievement in the pharmaceutical landscape, marking it as the first cannabis-derived medication sanctioned for medical use. This detailed examination explores the rigorous FDA approval process that Epidiolex underwent, emphasizing the methodical evaluations and regulatory requirements that facilitated its journey from an experimental drug to an approved treatment.
- Preclinical Research: Epidiolex was subjected to extensive preclinical research, including in vitro and in vivo studies, to evaluate its pharmacological behavior, toxicity, and initial safety profiles. These preclinical studies are vital for determining potential risks and establishing a safe dosing regimen before the drug is administered to humans.
- Investigational New Drug (IND) Application: Following favorable preclinical outcomes, GW Pharmaceuticals submitted an IND application to the FDA, which is necessary to begin clinical trials in humans. This application included detailed reports from preclinical studies, the drug’s manufacturing information, and the protocols for proposed clinical trials.
- Clinical Trial Phases: Epidiolex’s clinical development involved several critical phases:
- Phase I: Initial trials focused on evaluating the safety and pharmacological profile of Epidiolex in a small group of volunteers or patients.
- Phase II: These studies expanded to test the drug’s efficacy and safety in a larger group of patients who have the specific medical conditions targeted by the drug.
- Phase III: This phase involved extensive testing to confirm Epidiolex’s effectiveness, monitor adverse effects, and gather comparative data against existing treatments.
- New Drug Application (NDA) Submission: Successful clinical trials led to the submission of an NDA to the FDA by GW Pharmaceuticals. This submission is a critical step, compiling all data from the drug’s testing phases, proposed labels, detailed patient information, and manufacturing details, requesting FDA approval to market Epidiolex.
- FDA Review and Decision: The FDA’s evaluation of Epidiolex’s NDA was exhaustive, reviewing the clinical data, compliance with regulatory standards, manufacturing facility inspections, and the appropriateness of the drug’s labeling. This thorough review process culminated in the approval of Epidiolex in June 2018 for treating seizures associated with Dravet syndrome and Lennox-Gastaut syndrome.
- Post-Approval Monitoring: After approval, Epidiolex continues to be monitored under the FDA’s post-marketing surveillance program to ensure it remains safe and effective when used in a broader population. This includes tracking adverse reactions and conducting further studies to assess long-term effects.

In summary, the approval of Epidiolex demonstrates the rigorous and structured approach required by the FDA for a new drug to enter the market, ensuring it meets stringent standards for safety and efficacy. The sanctioning of Epidiolex not only provides a new therapeutic option for patients with severe epilepsy but also sets a regulatory framework for future approvals of cannabinoid-based medications.
Treating Epilepsy: How Epidiolex Helps with Seizures
Epilepsy is a complex neurological condition characterized by frequent seizures, presenting substantial treatment challenges, particularly for severe and rare forms.
Epidiolex, a cannabinoid-based medication sanctioned by the U.S. Food and Drug Administration (FDA), serves as a crucial treatment option, especially for conditions such as Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis complex. This document examines how Epidiolex assists in controlling seizures, details its clinical effectiveness, and discusses its role in contemporary epilepsy treatment strategies.
- Action Mechanism: The active ingredient in Epidiolex is cannabidiol (CBD), which operates distinctly from traditional antiepileptic drugs. CBD’s specific mechanisms of action in the brain are not entirely understood; however, it is believed to affect various brain targets that regulate neuronal excitability and seizure activities. Unlike typical antiepileptic agents that primarily target sodium channels or enhance GABA (the brain’s major inhibitory neurotransmitter) activity, CBD modulates the excitatory neurotransmitter glutamate and offers neuroprotective effects, thereby reducing neuronal overactivity and the spread of seizures.
- Effectiveness in Clinical Trials: Epidiolex’s ability to reduce seizure frequency has been substantiated through rigorous clinical trials. Studies involving patients with Lennox-Gastaut and Dravet syndromes have demonstrated that Epidiolex significantly lowers the frequency of seizures compared to a placebo. These results indicated a substantial improvement in patient quality of life. For patients with tuberous sclerosis complex, Epidiolex also effectively decreased the number of seizures, confirming its broad applicability as an antiepileptic medication.
- Administration and Dosage: Epidiolex is administered orally, with dosages adjusted based on patient weight to ensure personalized treatment. Initially prescribed at a lower dose, it is gradually increased to achieve optimal levels, balancing efficacy and side effects such as somnolence, reduced appetite, diarrhea, and increased liver enzymes.
- Incorporation into Treatment Plans: Epidiolex is often used alongside other antiepileptic medications, especially in cases where patients have not adequately responded to conventional treatments. This strategy is crucial for managing treatment-resistant epilepsy, providing a new pathway for seizure control. The adoption of Epidiolex into therapeutic regimens reflects a move towards more personalized medical treatments in epilepsy care.
- Implications for Regulatory and Medical Practice: The FDA’s approval of Epidiolex has been a critical milestone in epilepsy therapy, spotlighting the potential of cannabinoid therapies in neurological disorders. It has also encouraged ongoing research into cannabinoids, potentially expanding the range of therapeutic options available for epilepsy and other neurological conditions.
In summary, Epidiolex offers a novel and effective approach to managing some of the most challenging forms of epilepsy. Its unique action mechanism and proven success in clinical settings have established it as an invaluable addition to the epilepsy treatment arsenal, enhancing the lives of patients with this debilitating condition.
Dosage and Administration: Guidelines for Using Epidiolex
Epidiolex, which has received FDA approval for managing seizures linked to Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex, requires precise dosage and administration guidelines to ensure maximum therapeutic efficacy and safety. This document outlines the critical protocols for the proper administration of Epidiolex, tailored to individual patient needs.
- Determining the Correct Dosage: Dosage for Epidiolex is calculated based on the patient’s body weight, requiring careful adherence to weight-based dosing guidelines. Initially, patients should receive 2.5 mg/kg twice daily, totaling 5 mg/kg per day. One week following the initial administration, the dosage of Epidiolex should be escalated to a maintenance dose of 5 mg/kg administered twice daily, equating to 10 mg/kg per day. Based on the patient’s response and tolerance, this dosage may be further adjusted to a maximum of 10 mg/kg twice daily, totaling 20 mg/kg per day.
- Method of Administration: Epidiolex is supplied as an oral solution, which allows for easy administration, especially suitable for patients who may struggle with tablets. Accurate dosing should be measured using the provided syringe. The medication can be ingested with or without food; however, it is recommended to administer it consistently at meal times to maintain stable drug levels in the body.
- Adjusting Dosage for Optimal Results: If adverse effects such as drowsiness or gastrointestinal issues arise, it may be necessary to modify the dosage or the administration schedule. Careful titration of the dose can help manage side effects while preserving the drug’s beneficial effects.
- Continuous Monitoring and Management: Ongoing monitoring is essential for patients on Epidiolex, focusing on effectiveness, side effects, and overall health. Liver function tests should be conducted prior to initiating treatment and routinely monitored afterward, as elevated liver enzymes could indicate liver stress or damage. This monitoring is crucial during the initial phases of treatment and following any adjustments in dosage.
- Considerations for Specific Populations: Patients with existing liver impairments may require a reduced starting dose and slower dose increases. Detailed consultation of the prescribing information and guidance from a healthcare provider are recommended when managing dosage adjustments for patients with hepatic conditions.
- Guidance on Discontinuing Treatment: Should discontinuation of Epidiolex be necessary, gradually tapering the dose is recommended to reduce the risk of withdrawal symptoms or an increase in seizure frequency.
In summary, administering Epidiolex effectively requires stringent adherence to dosage guidelines based on patient weight and careful management of administration practices. Routine follow-up and dose adjustments, based on individual responses and health evaluations, are vital for achieving the best outcomes in epilepsy management with Epidiolex.

Side Effects and Safety Considerations of Epidiolex
Epidiolex, the first FDA-approved medication derived from cannabidiol (CBD), treats severe and rare forms of epilepsy, including Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex. Despite its therapeutic benefits, it is essential to be aware of its potential side effects and safety measures to ensure optimal patient care and treatment efficacy. This document delves into the side effects associated with Epidiolex, necessary monitoring protocols, and safety measures required for its administration.
- Common Side Effects: The administration of Epidiolex may lead to several side effects, the most frequent of which include drowsiness, decreased appetite, diarrhea, fatigue, and elevated liver enzymes. Other less common side effects may include insomnia, infections, rashes, and disturbed sleep. These effects generally range from mild to moderate severity and could influence patient adherence and overall quality of life.
- Liver Function Monitoring: A significant safety concern with Epidiolex is its potential to impair liver function, as evidenced by elevated liver enzymes in some patients, especially those concurrently taking other antiepileptic medications. It is recommended that liver function tests be performed before starting therapy and periodically thereafter to promptly identify any signs of liver injury.
- Drug Interaction Risks: Epidiolex may interact with other medications, potentially leading to heightened side effects or diminished therapeutic efficacy. It is known to interact with certain antiepileptic drugs, potentially requiring dosage adjustments or careful monitoring.
- Additionally, Epidiolex can influence the metabolic processing of other drugs, requiring adjustments to treatment regimens to ensure safe and effective use.
- Considerations for Specific Populations: Caution is advised when prescribing Epidiolex to particular groups, such as individuals with pre-existing liver conditions, where a reduced dose may be necessary to mitigate additional hepatic strain. Additionally, special attention should be paid to patients with a history of substance abuse or severe mood disorders, as there is a potential for exacerbation of these conditions.
- Side Effect Management: Addressing side effects from Epidiolex may involve adjusting the dosage, employing symptomatic treatments, or prescribing medications to manage specific symptoms, such as anti-nausea drugs for gastrointestinal complaints. This approach helps in managing side effects while maintaining the therapeutic integrity of the treatment plan.
- Ongoing Monitoring and Education: Continuous monitoring is crucial for those on Epidiolex to ensure safety and effectiveness. This includes routine health evaluations, tracking side effects, and maintaining adherence to prescribed doses. It is also crucial to educate patients and caregivers about potential side effects and the importance of adhering to treatment and monitoring schedules to ensure successful outcomes.
In summary, while Epidiolex offers significant benefits for treating certain complex epileptic disorders, recognizing and managing its potential side effects, and adhering to strict safety and monitoring protocols, are essential. By following established guidelines for dosage, monitoring, and managing interactions and side effects, healthcare providers can help maximize the benefits of Epidiolex for patients while minimizing associated risks.
Future Research and Developments: What’s Next for Epidiolex
As Epidiolex continues to revolutionize the management of severe and rare forms of epilepsy, the pathway ahead is ripe with opportunities for expanded clinical applications and enhancements in cannabinoid therapy. This document outlines prospective areas of research and development that could augment the effectiveness and applicability of Epidiolex within the healthcare sector.
- Broadening Clinical Applications: Key research initiatives are focusing on evaluating Epidiolex for potential use in additional neurological conditions beyond its approved applications for Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex. Early investigations into its effects on other neurological disorders marked by inflammation or autoimmune components suggest avenues for extended clinical trials aimed at widening its use.
- Advancements in Drug Formulation: Continued efforts are underway to refine the formulation of Epidiolex to improve patient adherence and minimize adverse effects. Future developments might include the creation of extended-release versions to simplify dosage schedules, enhance drug absorption, and mitigate gastrointestinal side effects, thereby enhancing patient experience and treatment outcomes.
- Personalized Medicine Through Genetic Research: Future studies are expected to explore genetic factors that could predict individual responses to Epidiolex, supporting a personalized approach to treatment. Research into biomarkers that could foresee efficacy and side effect profiles would allow clinicians to tailor treatments to individual patient needs, optimizing therapeutic results.
- Exploration of Combination Therapies: As Epidiolex becomes more integrated into diverse treatment regimens, investigating its combination with other antiepileptic drugs will be crucial. Such studies will assess the synergistic effects and safety of using Epidiolex in conjunction with other medications, aiming to maximize its therapeutic potential.
- Understanding Mechanisms of Action: There is a significant push to gain a deeper understanding of how Epidiolex works at a molecular level to control seizures. Continued research into how it pharmacologically interacts with the nervous system could pave the way for more targeted therapies that yield improved outcomes with fewer side effects.
- Navigating Regulatory and Market Changes: The regulatory environment will significantly influence the ongoing development and use of Epidiolex. Changes in cannabis regulation and policy, both within the U.S. and internationally, could affect its clinical and commercial availability. Moreover, the pharmaceutical market dynamics, including the introduction of generic versions post-patent expiration, will also impact its market presence and usage.
- Conducting Long-Term Impact Studies: Longitudinal research to evaluate the long-term safety and effectiveness of Epidiolex is essential. These studies will provide critical insights into the long-term impacts of the medication on liver health, cognitive function in pediatric patients, and its interactions with other chronic therapies.
In summary, the future of Epidiolex looks promising, with extensive potential for broadening its impact across various neurological disorders and improving cannabinoid-based medical treatments. Ongoing research and development will be key to leveraging its full potential, ensuring that it continues to provide significant benefits to patients with complex medical needs while advancing our understanding of cannabinoid therapies.