Table of Contents
Does marijuana help with inflammation
Marijuana, also known as cannabis, has long been used for both recreational and medicinal purposes. One of the most scientifically compelling areas of cannabis research involves its potential to reduce inflammation, which is a core component in many chronic illnesses and autoimmune disorders.
1. Understanding Inflammation
Inflammation is the body’s natural response to injury, pathogens, or irritants. While acute inflammation is protective and temporary helping to remove harmful stimuli and initiate healing chronic inflammation can be detrimental. Chronic inflammation is involved in diseases such as:
- Rheumatoid arthritis
- Inflammatory bowel disease (IBD)
- Multiple sclerosis (MS)
- Asthma
- Psoriasis
- Neurodegenerative diseases (e.g., Alzheimer’s)
- Cardiovascular disease
Because chronic inflammation is difficult to manage and often treated with drugs that have significant side effects, alternative therapies like cannabis are being studied intensively.
2. Cannabis and Its Active Components
The cannabis plant contains over 100 phytocannabinoids, the most prominent of which are:

- Tetrahydrocannabinol (THC): The main psychoactive component.
- Cannabidiol (CBD): A non-psychoactive cannabinoid with a broad range of therapeutic potential.
- Cannabigerol (CBG) and Cannabichromene (CBC): Less studied, but increasingly recognized for anti-inflammatory properties.
These cannabinoids interact with the body’s endocannabinoid system (ECS), a regulatory system involved in maintaining homeostasis across multiple physiological functions, including immune response and inflammation.
3. The Endocannabinoid System and Inflammation
The ECS is composed of:
- Endocannabinoids (e.g., anandamide and 2-AG)
- Cannabinoid receptors (CB1 and CB2)
- Enzymes that synthesize and degrade cannabinoids
CB1 receptors are primarily found in the brain and central nervous system, while CB2 receptors are abundant in the immune system, especially in immune cells like macrophages, T-cells, and microglia. CB2 receptor activation has been directly linked to anti-inflammatory effects.
When cannabinoids bind to these receptors:
- CB1 activation often modulates pain and mood.
- CB2 activation reduces the release of pro-inflammatory cytokines (like IL-6, TNF-alpha) and promotes anti-inflammatory signaling.
4. Cannabinoids with Anti-Inflammatory Properties
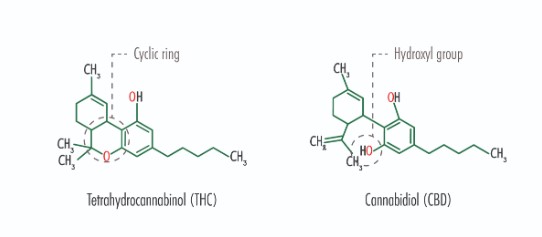
A. Cannabidiol (CBD)
CBD is particularly noteworthy for its broad anti-inflammatory action:
- It inhibits the production of cytokines and chemokines.
- Reduces oxidative stress by modulating reactive oxygen species (ROS).
- Influences TRPV1 (vanilloid) and PPAR-γ receptors, both of which play roles in inflammatory signaling.
- Suppresses NF-κB, a master regulator of inflammation.
B. Tetrahydrocannabinol (THC)
THC also has anti-inflammatory effects, mainly through CB2 receptor binding. It may:
- Inhibit T-cell proliferation.
- Reduce microglial activation in the brain.
- Lower levels of pro-inflammatory mediators like prostaglandins.
However, THC’s psychoactive effects can limit its clinical application, especially in chronic treatment scenarios.
C. Other Cannabinoids
- CBG: Inhibits COX-2 and iNOS enzymes involved in inflammation.
- CBC: Enhances anti-inflammatory response in the gastrointestinal tract and may contribute to synergistic effects when used with CBD and THC.
5. Preclinical and Clinical Study Evidence
Preclinical (Animal and Cell) Studies
Many rodent and cell culture studies have demonstrated cannabis’s anti-inflammatory effects:
- In models of colitis, CBD reduces intestinal inflammation and preserves epithelial barrier function.
- In arthritis models, both CBD and THC reduce joint swelling and immune cell infiltration.
- In neuroinflammation, cannabinoids suppress microglial activation and reduce brain tissue damage.
These studies provide a mechanistic foundation for therapeutic use, but translation to human treatments remains an active area of research.
Clinical Studies
Although fewer in number, human studies suggest beneficial effects in conditions characterized by inflammation:
- Multiple Sclerosis (MS): Nabiximols (a 1:1 THC/CBD spray) reduces spasticity and inflammation-related symptoms.
- Rheumatoid Arthritis: Some patients report reduced morning stiffness and joint pain with cannabinoid treatment.
- IBD (Crohn’s and Ulcerative Colitis): CBD-rich extracts have shown mixed results; some trials report symptom reduction, though others find no statistical significance.
- Fibromyalgia: A condition often associated with systemic inflammation, sees improvements in pain and fatigue in some cannabis users.
- Chronic Pain: Cannabis may reduce inflammation-induced sensitization, improving patient quality of life.
6. Anti-Inflammatory Mechanisms in Detail
Cannabinoids modulate inflammation through multiple pathways:
- NF-κB Inhibition: Cannabinoids block nuclear factor kappa B, which governs expression of inflammatory genes.
- Cytokine Modulation: They reduce levels of IL-1β, IL-6, and TNF-α—key players in the inflammatory cascade.
- Oxidative Stress Reduction: Cannabis enhances antioxidant defenses, lowering ROS and nitric oxide levels.
- Apoptosis of Immune Cells: Certain cannabinoids induce apoptosis in hyperactive immune cells, reducing autoimmune attack.
- Gut Microbiome Effects: Preliminary evidence suggests cannabis may stabilize gut microbiota, which plays a role in systemic inflammation.
7. Benefits Compared to Traditional Anti-Inflammatories
Conventional treatments like NSAIDs and corticosteroids are effective but come with risks:
- NSAIDs can cause gastrointestinal bleeding and kidney dysfunction.
- Steroids have systemic effects such as weight gain, immunosuppression, and mood alterations.
Cannabinoids, particularly CBD, offer:
- Lower risk of serious organ toxicity.
- Minimal psychoactive effects (for non-THC compounds).
- Multi-modal activity simultaneously reducing inflammation and modulating pain, mood, and sleep.
8. Challenges and Limitations
Despite promising findings, there are significant limitations:
- Regulatory Hurdles: Cannabis remains federally restricted in many countries, limiting research.
- Dosage Variability: Optimal dosing remains unclear. Too much THC, for example, can exacerbate anxiety or impair cognition.
- Strain Differences: Cannabis strains vary in cannabinoid and terpene content, leading to inconsistent effects.
- Long-Term Effects: More data is needed on chronic use and its impact on immune function, especially in vulnerable populations.
9. Role of Terpenes and the Entourage Effect
Terpenes like beta-caryophyllene, myrcene, and pinene have anti-inflammatory effects and may synergize with cannabinoids.
- Beta-caryophyllene is a dietary terpene that directly binds to CB2 receptors.
- The “entourage effect” refers to the synergy between cannabinoids and terpenes, potentially enhancing therapeutic outcomes.
10. Future Directions
To better understand and utilize cannabis for inflammation, future research must focus on:
- Strain-specific studies to correlate phytochemical profiles with anti-inflammatory effects.
- Precision medicine approaches, using biomarkers to personalize cannabinoid therapies.
- Comparative trials between cannabis-based therapies and standard anti-inflammatory drugs.
- Formulation optimization using standardized extracts or synthetic analogs like Epidiolex or Sativex.
Conclusion
Marijuana especially its non-psychoactive constituents like CBD shows substantial promise as an anti-inflammatory agent. Its effects are mediated through a complex network involving cannabinoid receptors, inflammatory cytokines, and oxidative stress pathways. While preclinical and some clinical evidence support its efficacy, larger-scale, controlled human studies are needed to validate its place in mainstream medicine. When used thoughtfully and under medical guidance, cannabis could offer a safer, more holistic alternative or adjunct to traditional anti-inflammatory therapies.